Power of protein/tRNA functional assembly against aberrant aggregation†
Abstract
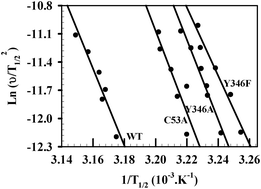
Understanding the mechanisms of protein oligomerization and aggregation is a major concern for biotechnology and medical purposes. However, significant challenges remain in determining the mechanism of formation of these superstructures and the environmental factors that can precisely modulate them. Notably the role that a functional ligand plays in the process of protein aggregation is largely unexplored. We herein address these issues with an original flavin-dependent RNA methyltransferase (TrmFO) used as a protein model since this protein employs a complex set of cofactors and ligands for catalysis. Here, we show that TrmFO carries an unstable protein structure that can partially mis-unfold leading to either formation of irregular and nonfunctional soluble oligomers endowed with hyper-thermal stability or large amorphous aggregates in the presence of salts. Mutagenesis confirmed that this peculiarity is an intrinsic property of a polypeptide and it is independent of the flavin coenzyme. Structural characterization and kinetic studies identified several regions of the protein that enjoy conformational changes and more particularly pinpointed the N-terminal subdomain as being a key element in the mechanisms of oligomerization and aggregation. Only stabilization of this region via tRNA suppresses these aberrant protein states. Although protein chaperones emerged as major actors against aggregation, our study emphasizes that other powerful mechanisms exist such as the stabilizing effect of functional assemblies that provide an additional layer of protection against the instability of the proteome.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...