Dissociative iodomethane adsorption on Ag-MOR and the formation of AgI clusters: an ab initio molecular dynamics study†
Abstract
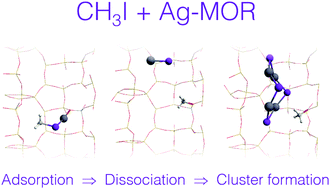
Radioactive iodine species belong to the most dangerous components of nuclear effluents and waste produced by nuclear facilities. In this work, we use computer simulations at the periodic DFT level to investigate dissociative adsorption of iodomethane on silver-exchanged mordenite, which is among the most effective sorbents of iodine species available today. The structure, energetics, and mobility of complexes Ag–(CH3I) and Ag–(CH3I)2 formed upon adsorption of iodomethane on Ag+ sites are investigated using the ab initio MD approach. The free-energy profiles for the reaction CH3I + Ag-MOR → AgI + CH3-MOR are determined using the blue moon ensemble technique. The AgI species formed as a product of dissociative adsorption are shown to combine spontaneously into small clusters (AgI)n with the dimensions restricted by the size and geometry of confining voids. The structure and energetics of the (AgI)n species are analysed in detail and compared with the available experimental and theoretical data. The internal energy of formation of clusters in mordenite is shown to contribute significantly to the shift of equilibrium from the undissociated to dissociated form of adsorbed CH3I.


 Please wait while we load your content...
Please wait while we load your content...