The role of the σ-holes in stability of non-bonded chalcogenide⋯benzene interactions: the ground and excited states†
Abstract
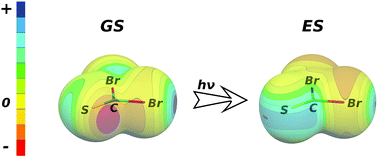
The stability of the T-shaped and stacked complexes of benzene with methanethial (CH2S) and methaneselone (CH2Se) and their difluoro-, dichloro-, dibromo-derivatives is investigated in their ground and first electronic excited states by means of the SCS-ADC2 method. The origin of the stabilization in the ground state is discussed based on the results of calculations performed using the DFT-SAPT method. Calculations show that the stability of the T-shaped conformers increases upon electronic excitation, while it decreases for most of the stacked conformers. Both effects are explained by the changes in the electrostatic potential (ESP) of isolated monomers upon the electronic excitation.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...