Effects of H+ and OH− on H2O as probed by the 1-propanol probing methodology: differential thermodynamic approach†
Abstract
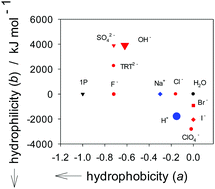
We applied what we call the “1-propanol (1P) probing methodology” on the effects of H+ and OH− on liquid H2O. We found that H+ is an amphiphile with a modest hydrophobic and an equally modest hydrophilic contribution. Its hydration number is 2 ± 1, suggesting that the equilibrium hydration structure is like the Zundel type (H5O2+). OH−, on the other hand, has a large hydration shell with 12 ± 3 H2O molecules and acts as a hydrophobe-like hydration center. In other words, it forms a hydration shell around itself, but as the probing 1P increases and the available H2O decreases, it exerts its influence over a longer range and reduces the hydrogen-bond probability of bulk H2O away from hydration shells, just as a hydrophobe does to bulk H2O.


 Please wait while we load your content...
Please wait while we load your content...