Isomerization of methoxy radical in the troposphere: competition between acidic, neutral and basic catalysts†
Abstract
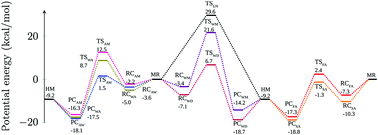
A comprehensive investigation of the roles of acidic, neutral and basic catalysts in isomerization of methoxy radical in the troposphere has been carried out by quantum chemical calculations at the MP2 and CCSD(T) levels of theory. The effect of basic catalysts, namely ammonia and an ammonia–water complex, on the isomerization process has been studied for the very first time. In terms of rate coefficients ammonia was found to be a better catalyst than a water monomer whereas the ammonia–water complex was found to be more efficient over a water dimer but marginally less efficient than formic acid. Based on the effective rate constants under various tropospheric conditions, it was found that at 0 km altitude water dimers and ammonia–water complexes could compete with acid catalysts but at higher altitudes the acid catalysts would dominate their neutral and basic counterparts by a long distance.


 Please wait while we load your content...
Please wait while we load your content...