Non-contact electric potential measurements of electrode components in an operating polymer electrolyte fuel cell by near ambient pressure XPS†
Abstract
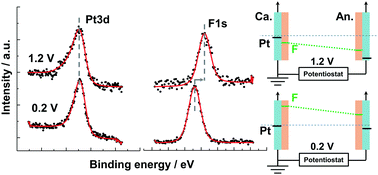
Photoelectron spectroscopy has the advantage of providing electric potentials by non-contact measurements based on the kinetic energy shift in component potential. We performed operando hard X-ray photoelectron spectroscopy (HAXPES) measurements with an 8 keV excitation source to measure the shift in electron kinetic energies as a function of the voltages of all the components at the anode and cathode electrodes of a polymer electrolyte fuel cell (PEFC). At the cathode electrode, when we increase the voltage between the cathode and anode from 0.2 to 1.2 V, the O 1s and F 1s peaks shift to a lower binding energy and the magnitude of the energy shift is equal to the voltage. The Pt 3d and C 1s peaks do not shift with the voltage since platinum nanoparticles and carbon supports at the cathode electrode have ground contact. In contrast to the cathode electrode, the peak shifts of all the components at the anode electrode show the same amount of shift as the voltages. It is clear that the change in the potential difference occurs only in an electrical double layer at the interface between the cathode electrode (Pt/C) and the electrolyte (Nafion and water), and that the anode electrode is in equilibrium as a pseudo-hydrogen electrode. Moreover, the electric potential variation of the cathode electrode in a PEFC under a power generation condition was also directly detected by operando HAXPES.


 Please wait while we load your content...
Please wait while we load your content...