Noble gas bond and the behaviour of XeO3 under pressure
Abstract
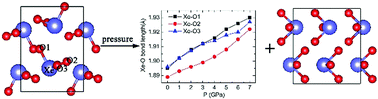
Over the past few decades, the concept of hydrogen bonds, in which hydrogen is electrophilic, has been extended to halogen bonds, chalcogen bonds and pnicogen bonds. Herein, we show that such a non-covalent bonding also exists in noble gas compounds. Using first principles calculations, we illustrate the O⋯Xe–O bond in molecular crystal XeO3 and its effect on the behavior of this compound under pressure. Our calculations show that the covalent Xe–O bond lengths were elongated with increasing pressure and correspondingly the Xe–O stretching vibration frequencies were red shifted, which is similar to the change of H-bonds under pressure. The O⋯Xe–O bond and related hopping of O between neighboring Xe sites also correspond to the structural changes in the XeO3 compounds at about 2 GPa. Our study extends the concept of hydrogen bonding to include all p-block elements and show a new bonding type for Noble gas elements in which it acts as an electrophilic species.


 Please wait while we load your content...
Please wait while we load your content...