Quantum chemical investigation of the thermal denitrogenation of 1-pyrazoline†
Abstract
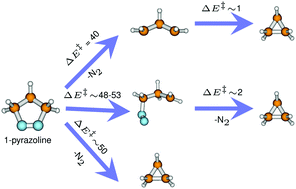
Understanding the mechanism of the denitrogenation of 1-pyrazolines is of fundamental importance due to the unusual stereochemical preferences (major single inversion) seen in the product formation. In the present work, a detailed investigation on the mechanisms of the thermal denitrogenation of 1-pyrazoline was undertaken using CASSCF and CASPT2 methods with 6-31+G*, 6-311+G*, cc-pVDZ, cc-pVTZ, and aug-cc-pVDZ basis sets. The CASSCF calculations were performed with a series of different active spaces. It was found that the energetics obtained from CASSCF(4,4), where the σ,σ* orbitals of both the C–N bonds were included in the active space, are similar to those obtained using the (12,12) active space. The CASSCF(4,4) energetics were found to remain unaffected with the increase in the basis sets. Three different denitrogenation paths were obtained: (1) a synchronous path where a simultaneous breaking of both the C–N bonds leads to a planar trimethylene diradical intermediate, (2) an asynchronous concerted path which involves the unsymmetrical breaking of C–N σ bonds resulting in single inverted cyclopropane formation, and (3) an asynchronous step-wise path which involves the unsymmetrical breaking of the C–N bonds leading to diazenyl diradical intermediates. The barrier for the synchronous denitrogenation path was found to be lower in energy than that of the asynchronous paths. To check the applicability of the DFT and MP2 methods for this reaction, the potential energy profiles were mapped using different DFT functionals (B3LYP, B2PLYP, M06-2X) and the MP2 method. However, the DFT and MP2 methods failed to provide a correct description of the potential energy surface in the diradical regions.


 Please wait while we load your content...
Please wait while we load your content...