Full dimensional potential energy surface and low temperature dynamics of the H2CO + OH → HCO + H2O reaction
Abstract
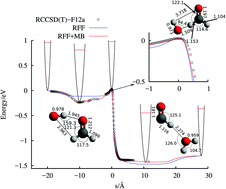
A new method is proposed to analytically represent the potential energy surface of reactions involving polyatomic molecules capable of accurately describing long-range interactions and saddle points, needed to describe low-temperature collisions. It is based on two terms, a reactive force field term and a many-body term. The reactive force field term accurately describes the fragments, long-range interactions among them and the saddle points for reactions. The many-body term increases the desired accuracy everywhere else. This method has been applied to the OH + H2CO → H2O + HCO reaction, giving a barrier of 27.4 meV. The simulated classical rate constants with this potential are in good agreement with recent experimental results [Ocaña et al., Astrophys. J., 2017, submitted], showing an important increase at temperatures below 100 K. The reaction mechanism is analyzed in detail here, and explains the observed behavior at low energy by the formation of long-lived collision complexes, with roaming trajectories, with a capture observed for very long impact parameters, >100 a.u., determined by the long-range dipole–dipole interaction.
- This article is part of the themed collection: Theory, experiment, and simulations in laboratory astrochemistry


 Please wait while we load your content...
Please wait while we load your content...