Solvation of apolar compounds in protic ionic liquids: the non-synergistic effect of electrostatic interactions and hydrogen bonds†
Abstract
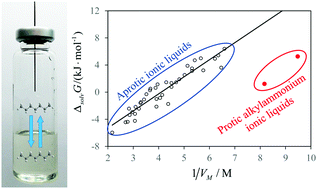
The solvation properties of protic ionic liquids such as alkylammonium salts are still virtually uncharacterized. Both electrostatic interactions between charged particles and hydrogen bond networks in a solvent are known to hinder the solubility of apolar species. Protic ionic liquids can be a priori expected to dissolve hydrocarbons worse than aprotic ionic liquids which do not form hydrogen bonds between the ions. We measured the limiting activity coefficients of several alkanes and alkylbenzenes in propylammonium and butylammonium nitrates at 298 K. Surprisingly, we observed the tendency of higher solubility than for the same compounds in aprotic ionic liquids with a similar molar volume. The calculations of the excess Gibbs free energies using test particle insertions into the snapshots of molecular dynamics trajectories reproduced lower values in protic rather than in aprotic ionic liquids for both methane molecules and hard sphere solutes. This can be explained by the favorable solvation of apolar species in the apolar domain of nanostructured PILs. For the first time, we point out at the essential difference between the solvation properties of two types of ionic liquids and prove that it arises from the cavity formation term.


 Please wait while we load your content...
Please wait while we load your content...