On the interfacial charge transfer between solid and liquid Li+ electrolytes
Abstract
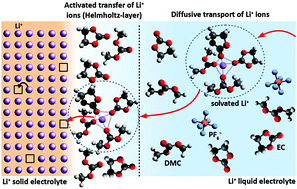
The Li+ ion transfer between a solid and a liquid Li+ electrolyte has been investigated by DC polarisation techniques. The current density i is measured as a function of the electrochemical potential drop Δ![[small mu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e0.gif) Li+ at the interface, using a liquid electrolyte with different Li+ concentrations. The subject of this experimental study is the interface between the solid electrolyte Ta-substituted lithium lanthanum zirconate (Li6.6La3Zr1.6Ta0.4O12) and a liquid electrolyte consisting of LiPF6 dissolved in ethylene carbonate/dimethyl carbonate (1 : 1). The functional course of i vs. Δ
Li+ at the interface, using a liquid electrolyte with different Li+ concentrations. The subject of this experimental study is the interface between the solid electrolyte Ta-substituted lithium lanthanum zirconate (Li6.6La3Zr1.6Ta0.4O12) and a liquid electrolyte consisting of LiPF6 dissolved in ethylene carbonate/dimethyl carbonate (1 : 1). The functional course of i vs. Δ![[small mu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e0.gif) Li+ can be described by a serial connection between a constant ohmic resistance Rslei and a current dependent thermally activated ion transfer process. For the present solid–liquid electrolyte interface the areal resistance Rslei of the surface layer is independent of the Li+ concentration in the liquid electrolyte. At room temperature a value of about 300 Ω cm2 is found. The constant ohmic resistance Rslei can be attributed to a surface layer on the solid electrolyte with a (relatively) low conductivity (solid–liquid electrolyte interphase). The low conducting surface layer is formed by degradation reactions with the liquid electrolyte. Rslei is considerably increased if a small amount (ppm) of water is added to the liquid electrolyte. The thermally activated ionic transfer process obeys a Butler–Volmer like behaviour, resulting in an exchange current density i0 depending on the Li+ concentration in the liquid electrolyte by a power-law. At a Li+ concentration of 1 mol l−1 a value of 53.1 μA cm−2 is found. A charge transfer coefficient α of ∼0.44 is measured. The finding of a superposed constant ohmic resistance due to a solid–liquid electrolyte interphase and a current dependent thermally activated ion transfer process is confirmed by the results of two former experimental studies from the literature, performing AC measurements/EIS.
Li+ can be described by a serial connection between a constant ohmic resistance Rslei and a current dependent thermally activated ion transfer process. For the present solid–liquid electrolyte interface the areal resistance Rslei of the surface layer is independent of the Li+ concentration in the liquid electrolyte. At room temperature a value of about 300 Ω cm2 is found. The constant ohmic resistance Rslei can be attributed to a surface layer on the solid electrolyte with a (relatively) low conductivity (solid–liquid electrolyte interphase). The low conducting surface layer is formed by degradation reactions with the liquid electrolyte. Rslei is considerably increased if a small amount (ppm) of water is added to the liquid electrolyte. The thermally activated ionic transfer process obeys a Butler–Volmer like behaviour, resulting in an exchange current density i0 depending on the Li+ concentration in the liquid electrolyte by a power-law. At a Li+ concentration of 1 mol l−1 a value of 53.1 μA cm−2 is found. A charge transfer coefficient α of ∼0.44 is measured. The finding of a superposed constant ohmic resistance due to a solid–liquid electrolyte interphase and a current dependent thermally activated ion transfer process is confirmed by the results of two former experimental studies from the literature, performing AC measurements/EIS.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...