Structural transition in metal-centered boron clusters: from tubular molecular rotors Ta@B21 and Ta@B22+ to cage-like endohedral metalloborospherene Ta@B22−†
Abstract
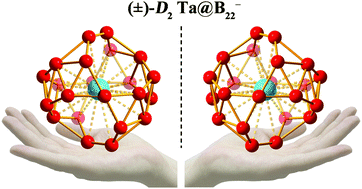
Inspired by the recent discovery of the metal-centered tubular molecular rotor Cs B2-Ta@B18− with the record coordination number of CN = 20 and based on extensive first-principles theory calculations, we present herein the possibility of the largest tubular molecular rotors Cs B3-Ta@B18 (1) and C3v B4-Ta@B18+ (2) and smallest axially chiral endohedral metalloborospherenes D2 Ta@B22− (3 and 3′), unveiling a tubular-to-cage-like structural transition in metal-centered boron clusters at Ta@B22−via effective spherical coordination interactions. The highly stable Ta@B22− (3) as an elegant superatom, which features two equivalent corner-sharing B10 boron double chains interconnected by two B2 units with four equivalent B7 heptagons evenly distributed on the cage surface, conforms to the 18-electron configuration with a bonding pattern of σ + π double delocalization and follows the 2(n + 1)2 electron counting rule for spherical aromaticity (n = 2). Its calculated adiabatic detachment energy of ADE = 3.88 eV represents the electron affinity of the cage-like neutral D2 Ta@B22 which can be viewed as a superhalogen. The infrared, Raman, VCD, and UV-vis spectra of the concerned species are computationally simulated to facilitate their spectral characterizations.


 Please wait while we load your content...
Please wait while we load your content...