Effect of donor to acceptor ratio on electrochemical and spectroscopic properties of oligoalkylthiophene 1,3,4-oxadiazole derivatives†
Abstract
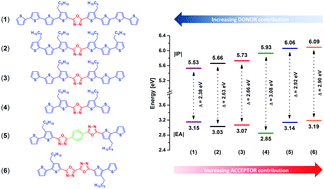
A structure–property study across a series of donor–acceptor–donor structures composed of mono- and bi-(1,3,4-oxadiazole) units symmetrically substituted with alkyl functionalized bi-, ter- and quaterthiophene segments is presented. Synthetically tailoring the ratio of electron-withdrawing 1,3,4-oxadiazole to electron-releasing thiophene units and their alkyl grafting pattern permitted us to scrutinize the impact of these structural factors on the redox, absorptive and emissive properties of these push–pull molecules. Contrasting trends of redox potentials were observed, with the oxidation potential closely following the donor-to-acceptor ratio, whereas the reduction potential being tuned independently by either the number of acceptor units or the conjugation length of the donor–acceptor system. Increasing the thiophene unit contribution delivered a shift from blue to green luminescence, while the structural rigidity afforded by intramolecular non-covalent interactions between 1,3,4-oxadiazole and the thiophene moieties has been identified as the prime factor determining the emission efficiency of these molecules. All six structures investigated electro-polymerize easily, yielding electroactive and electrochromic polymers. The polymer doping process is largely influenced by the length of the oligothiophene repeating unit and the alkyl chain grafting density. Polymers with relatively short oligothiophene segments are able to support polarons and polaron-pairs, whereas those with segments longer than six thiophene units could also stabilize diamagnetic charge carries – bipolarons. Increasing the alkyl chain grafting density improved the reversibility and broadened the working potential window of the p-doping process. Stable radical anions have also been investigated, bringing detailed information about the conjugation pattern of these electron-surplus species. This study delivers interesting clues towards the conscious structural design of bespoke frontier energy level oligothiophene functional materials and their polymers by incorporating a structurally matching 1,3,4-oxadiazole unit.


 Please wait while we load your content...
Please wait while we load your content...