Nonradiative dynamics determined by charge transfer induced hydrogen bonding: a combined femtosecond time-resolved fluorescence and density functional theoretical study of methyl dimethylaminobenzoate in water†
Abstract
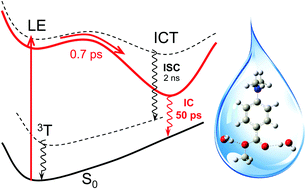
As a case study of the interplay and the consequence of the interplay between intramolecular charge transfer (ICT) and intermolecular hydrogen (H)-bonding, a combined femtosecond time-resolved fluorescence (fs-TRF) and density functional theoretical (DFT) and time-dependent DFT (TDDFT) study has been conducted on methyl dimethylaminobenzoate (MDMABA) largely in a water solvent. Direct observation of the broadband spectra, anisotropy, and kinetic decays of fs-TRF from photo-excited MDMABA revealed a rapid ICT reaction occurring with a time constant of ∼0.7 ps from an initial locally excited (LE) state identified to have the Laππ* character; this produced a weakly emissive ICT state featuring radiative rate constant decreased by more than two orders of magnitude. The fluorescence of the ICT state is strongly quenched exhibiting a decay time of ∼49.7 ps, unusually faster than the nanosecond range lifetime in a polar aprotic solvent when intersystem crossing (ISC) is the major deactivation channel. This, according to the study of the solvent kinetic isotope effect, is identified to originate from an instantly enhanced strong solute–solvent H-bonding induced by the ICT reaction which allows elimination of the ISC, and enables the nonradiative decay to proceed almost entirely through the otherwise inaccessible internal conversion from the ICT state. The enhancement of H-bonding is verified by the calculation which presents theoretical evidence for not only the binding site and binding energy of the H-bonding configuration but also the electronic and structural characterization, lending support to the twisted ICT (TICT) description of the photo-excited MDMABA. This study contributes a prominent example for the extraordinary ability of water and a decisive role of ICT promoted H-bonding in offering a highly effective molecular mechanism for rapid elimination of the electronic excitation energy. The results contain an important insight for the in-depth understanding of the excited state H-bonding dynamics, and also have significant implication for clarifying the “sunscreen controversy” of the DMABA type of UVB sunscreen molecule.


 Please wait while we load your content...
Please wait while we load your content...