At the crossroad of photochemistry and radiation chemistry: formation of hydroxyl radicals in diluted aqueous solutions exposed to ultraviolet radiation†
Abstract
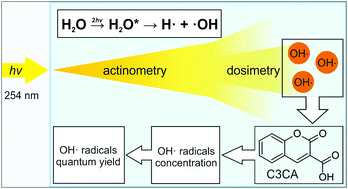
Formation yields of ˙OH radicals were precisely determined in aqueous solutions of coumarin-3-carboxylic acid and ferrous sulfate (i.e., Fricke dosimeter) exposed to 253.7 nm radiation delivered from a continuous source. Quantum yield of ˙OH radicals was determined as ∼0.08, i.e., roughly one out of twelve photons, efficiently absorbed in UV-illuminated solutions, produced one ˙OH radical. Energetically, a water molecule should undergo a correlated action of at least two 4.9 eV photons delivering enough energy for direct H–OH dissociation (5.0–5.4 eV). We suggest a mechanism based on an interaction of two water molecules, both in long-living triplet states. An intermolecular transfer of excitation energy provided a sufficient amount of energy for the dissociation of one water molecule into ˙OH and H˙ radicals. In an aqueous solution of phospholipids, quantum yields of hydroperoxides formed under these irradiation conditions decreased with total effectively absorbed energy (i.e. a dose), similar to the radiation chemical yields obtained during an exposure to ionizing radiation, such as gamma rays from radionuclide sources. Under 253.7 nm irradiation, one ˙OH radical causes a peroxidation of 34 phospholipid molecules. This implicates chain mechanism of the reaction.


 Please wait while we load your content...
Please wait while we load your content...