Solid state vibrational circular dichroism towards molecular recognition: chiral metal complexes intercalated in a clay mineral†
Abstract
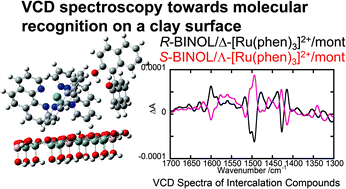
Vibrational circular dichroism (VCD) spectroscopy was applied to study chirality recognition in the interlayer space of a clay mineral. Clay intercalation compounds including two kinds of chiral molecules were prepared. Firstly a cationic metal complex, Δ- or Λ-[Ru(phen)3]2+ (phen = 1,10-phenanthroline), was ion-exchanged into sodium montmorillonite. Thereafter a neutral organic molecule, R- or S-1,1′-bi-2-naphthol (denoted as R- or S-BINOL), was co-adsorbed. The solid state VCD spectra were recorded on the hybrid compounds thus prepared. The intensity of VCD peaks in the region of 1300–1400 cm−1, which were assigned to the bending vibrations of OH groups in BINOL, was remarkably dependent on the chirality relation between the two intercalated species. This implied that BINOL took a different conformation in response to the chirality of co-existing [Ru(phen)3]2+.
- This article is part of the themed collections: 2018 PCCP HOT Articles and Complex molecular systems: supramolecules, biomolecules and interfaces


 Please wait while we load your content...
Please wait while we load your content...