A comparison of the solvation structure and dynamics of the lithium ion in linear organic carbonates with different alkyl chain lengths†
Abstract
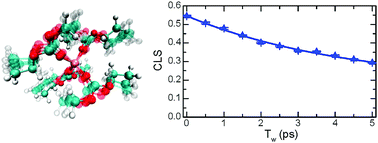
The structure and dynamics of electrolytes composed of lithium hexafluorophosphate (LiPF6) in dimethyl carbonate, ethyl methyl carbonate, and diethyl carbonate were investigated using a combination of linear and two-dimensional infrared spectroscopies. The solutions studied here have a LiPF6 concentration of X(LiPF6) = 0.09, which is typically found in commercial lithium ion batteries. This study focuses on comparing the differences in the solvation shell structure and dynamics produced by linear organic carbonates of different alkyl chain lengths. The IR experiments show that either linear carbonate forms a tetrahedral solvation shell (coordination number of 4) around the lithium ion irrespective of whether the solvation shell has anions in close proximity to the carbonates. Moreover, analysis of the absorption cross sections via FTIR and DFT computations reveals a distortion in the angle formed by Li+–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C which decreases from the expected 180° when the alkyl chains of the carbonate are lengthened. In addition, our findings also reveal that, likely due to its asymmetric structure, ethyl methyl carbonate has a significantly more distorted tetrahedral lithium ion solvation shell than either of the other two investigated carbonates. IR photon echo studies further demonstrate that the motions of the solvation shell have a time scale of a few picoseconds for all three linear carbonates. Interestingly, a slowdown of the in place-motions of the first solvation shell is observed when the carbonate has a longer alkyl chain length irrespective of the symmetry. In addition, vibrational energy transfer with a time scale of tens of picoseconds is observed between strongly coupled modes arising from the solvation shell structure of the Li+ which corroborates the modeling of these solvation shells in terms of highly coupled vibrational states. Results of this study provide new insights into the molecular structure and dynamics of the lithium ion electrolyte components as a function of solvent structure.
C which decreases from the expected 180° when the alkyl chains of the carbonate are lengthened. In addition, our findings also reveal that, likely due to its asymmetric structure, ethyl methyl carbonate has a significantly more distorted tetrahedral lithium ion solvation shell than either of the other two investigated carbonates. IR photon echo studies further demonstrate that the motions of the solvation shell have a time scale of a few picoseconds for all three linear carbonates. Interestingly, a slowdown of the in place-motions of the first solvation shell is observed when the carbonate has a longer alkyl chain length irrespective of the symmetry. In addition, vibrational energy transfer with a time scale of tens of picoseconds is observed between strongly coupled modes arising from the solvation shell structure of the Li+ which corroborates the modeling of these solvation shells in terms of highly coupled vibrational states. Results of this study provide new insights into the molecular structure and dynamics of the lithium ion electrolyte components as a function of solvent structure.


 Please wait while we load your content...
Please wait while we load your content...