A study of the competitive multiple hydrogen bonding effect and its associated excited-state proton transfer tautomerism†
Abstract
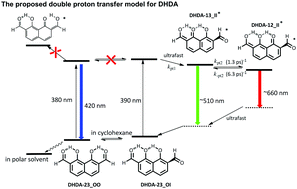
1,8-Dihydroxynaphthalene-2,7-dicarbaldehyde (DHDA) has been strategically designed and synthesized with the aim to study the competitive multiple hydrogen bonding (H-bonding) effect and the associated excited-state intramolecular proton transfer reaction (ESIPT). In nonpolar solvents such as cyclohexane, equilibrium exists between the two H-bonding isomers DHDA-23_OO and DHDA-23_OI, both of which possess double intramolecular H-bonds. In polar, aprotic solvents such as CH2Cl2, DHDA-23_OO becomes the predominant species. Due to various degrees of H-bond induced changes of electronic configuration each isomer reveals a distinct absorption feature and excited-state behavior, in which DHDA-23_OI in cyclohexane undergoes double ESIPT in a stepwise manner, giving the first and second proton-transfer tautomer emissions maximized at ∼500 nm and 660 nm, respectively. As for DHDA-23_OO both single and double ESIPT are prohibited, resulting in an intense normal 450 nm emission band. In a single crystal DHDA-23_OI is the dominant species, which undergoes excited state double proton transfer, giving intense emission bands at 530 nm and 650 nm. The mechanism associated with competitive multiple H-bonding energetics and ESIPT was underpinned by detailed spectroscopy/dynamics and computational approaches.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...