Enhanced intersystem crossing in carbonylpyrenes†
Abstract
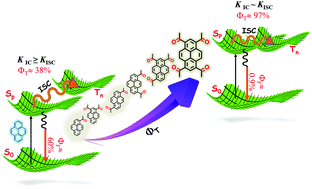
Ultrafast intersystem crossing of carbonylpyrenes in chloroform was investigated by femtosecond pump–probe spectroscopy. When compared to the dominant fluorescence decay pathway in pyrene, carbonyl functionalized pyrenes display near-unity triplet formation upon photoexcitation. The excited singlet state (Sp) undergoes rapid intersystem crossing (kISC) concomitantly with internal conversion (kIC) to lower excited singlet states (Sn) within a timescale of 5–11 ps (1/τ2 = kIC + kISC). Furthermore, intersystem crossing from lower excited singlet states (Sn) proceeds through coupling with receiver triplet states, eventually leading to high triplet quantum yields (ΦT = 97%; tetraacetylpyrene). Followed by internal conversion in the triplet manifolds, phosphorescence decay on a microsecond timescale is observed from the emitter triplet state.


 Please wait while we load your content...
Please wait while we load your content...