Catalytic reduction of SO2 by CO over Au4Pt2(CO)n and Au6Pt(CO)n clusters: a first-principles study
Abstract
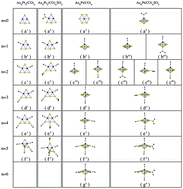
The catalytic properties of the magic gold–platinum bimetallic clusters (Au4Pt2 and Au6Pt) for the reduction of SO2 by CO, without or with preadsorbing CO molecules, are firstly investigated using density functional theory calculations. We find that the catalytic activities improve effectively with the preadsorption of CO onto the catalysts and that the catalytic activities of Au6Pt(CO)n are better than those of Au4Pt2(CO)n as more CO molecules are adsorbed onto the catalysts. During the reaction process, the Au4Pt2(CO)n clusters always keep two-dimensional morphologies except for when n = 5 and the Au6Pt(CO)n clusters have three-dimensional geometries except for when n = 0. The most stable adsorption site for SO2 molecules on the catalysts is the site of preadsorbing the next CO molecule on the corresponding catalysts. The largest activation energy (Emaxa) is related to the metal 5d (M-5d) band center and the charge transfer (Ct) as well as the bond length (Rb) between COS and the catalyst contribute to the desorption energy (Ed) of COS corporately. We propose that Au6Pt(CO)6 is a cost-effective gold–platinum bimetallic catalyst for the reduction of SO2 by CO.


 Please wait while we load your content...
Please wait while we load your content...