Stimuli-responsive NLO properties of tetrathiafulvalene-fused donor–acceptor chromophores†
Abstract
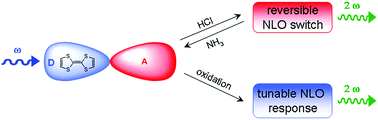
The second-order nonlinear optical (NLO) properties of two tetrathiafulvalene (TTF)-fused electron donor–acceptor dyads have been determined using the Electric Field Induced Second Harmonic generation (EFISH) technique and theoretically rationalized. Dyads TTF-dppz (1) and TTF-BTD (2) were obtained by direct fusion of a TTF electron donor unit either with a dipyrido[3,2-a:2′,3′-c]phenazine (dppz) or a benzothiadiazole (BTD) electron acceptor moiety. Dyad 1 acts as a reversible acido-triggered NLO switch by protonation/deprotonation at two nitrogen atoms of the dppz acceptor moiety induced by sequential exposure to HCl and ammonia vapors. Dyad 2, on the other hand, displays redox-tunable NLO properties upon two consecutive oxidations to its radical cation 2+˙ and dication 22+ species. The resulting final dication 22+ exhibits an inversion of the sign of β0, due to a completely inverted distribution of the frontier molecular orbitals with respect to those of its neutral species, leading to a scarcely polar species in the excited state, as indicated by the theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...