Weak C–H⋯F–C hydrogen bonds make a big difference in graphane/fluorographane and fluorographene/fluorographane bilayers
Abstract
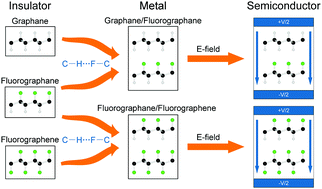
Using density functional theory computations with van der Waals (vdW) corrections, we reveal that C–H⋯F–C hydrogen bonding exists in graphane/fluorographene and fluorographane/fluorographane bilayers. The significant C–H⋯F–C hydrogen bonding is strong enough to combine two separate monolayers to form the bilayer. Interestingly, both the graphane/fluorographene and fluorographane/fluorographane bilayers are metallic in the most stable stacking configuration. Applying a perpendicular electric field can effectively open a bandgap for both bilayers, and we found that the field-induced gap opening for both graphane/fluorographene and fluorographane/fluorographane bilayers can be enhanced by biaxial tensile strain. These results are expected to aid in the design of novel electronic and optoelectronic devices based on graphene materials, and they highlight the use of weak interactions for modulating band structures in two-dimensional materials.


 Please wait while we load your content...
Please wait while we load your content...