A DFT study on the aldol condensation reaction on MgO in the process of ethanol to 1,3-butadiene: understanding the structure–activity relationship†
Abstract
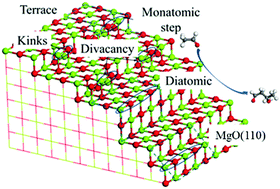
Using periodic density functional theory calculations, the aldol condensation of acetaldehyde to 3-hydroxybutanal over dehydroxylated MgO surfaces with and without structure defects was investigated. Compared with the C–C coupling step, the enolization step via proton transfer of the α-hydrogen of acetaldehyde to the MgO surface or the proton back-transfer step to form the desired 3-hydroxybutanal has a higher energy barrier, indicating that the proton transfer process is the key step for the aldol condensation on MgO. To highlight the effect of water, we also calculated the proton transfer steps in the presence of water and studied the reaction pathways over the partially hydroxylated MgO surface. The results show that water can participate in the proton back-transfer step by donating a proton to the alkoxide anion to form the 3-hydroxybutanal, thus reducing the activation energy; the surface OH groups induce a lowering of the activation energy barriers for the overall reaction. The results of the electronic structure analysis indicate that a strong Lewis acid–weak/medium base pair may have the best performance for aldol condensation, such as Mg3C–O4C-D produced by divacancy defects and Mg4C–O2CH produced by the dissociative adsorption of water. A strong Lewis acid generated by low-coordinated Mg2+ can adsorb and stabilize the acetaldehyde molecule near the catalyst surface which is beneficial for the abstraction of an α-proton from an acetaldehyde molecule, and a medium or weak Brønsted base is favorable for the proton back-transfer step.


 Please wait while we load your content...
Please wait while we load your content...