Symmetry and dynamics of FHF− anion in vacuum, in CD2Cl2 and in CCl4. Ab initio MD study of fluctuating solvent–solute hydrogen and halogen bonds†
Abstract
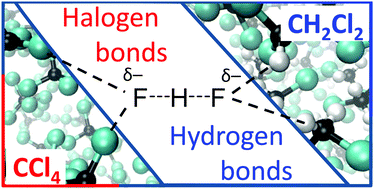
FHF− anion is a classic example of a central-symmetric strongly hydrogen bonded system that has been intensively investigated both experimentally and theoretically. In this paper we focus on solvent effects on symmetry, structure and dynamics of the anion. The FHF− anion is studied in vacuum, dissolved in CH2Cl2 and dissolved in CCl4 by ab initio molecular dynamics simulations. We show that CH2Cl2 molecules form CH⋯F hydrogen bonds with lone pairs of fluorine atoms, while CCl4 molecules form halogen bonds. These specific non-covalent solvent–solute interactions are cooperatively coupled to the FHF− hydrogen bonds. The fluctuation of solvent molecules’ positions is the driving force changing the FHF− hydrogen bond geometry. Most of the time, the anion is solvated asymmetrically, which leads to the asymmetric bridging particle position, though the time-averaged D∞h symmetry is retained. Interestingly, this transient asymmetrization of FHF− is more pronounced in CCl4, than in CH2Cl2. We show that the 1H and 19F NMR chemicals shifts react sensitively to the changes of anion's geometry and the limiting chemical shifts of free solvated FH and F− are strongly solvent-dependent.


 Please wait while we load your content...
Please wait while we load your content...