Physicochemical and electrochemical characterisation of imidazolium based IL + GBL mixtures as electrolytes for lithium-ion batteries†
Abstract
Ionic liquid/organic solvent mixtures are investigated as potential optimal electrolytes for lithium-ion batteries (LIBs) that can combine low flammability, good thermal stability and high electrical conductivity. In this work the standard ionic association constants 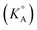 of different 1-alkyl-3-methylimidazolium ([Cnmim]+, n is the number of C in the alkyl side chain – 2, 4, 6 or 8) TFSI− based ionic liquids (ILs) in γ-butyrolactone (GBL) are determined using the low concentration Chemical Model (lcCM). Based on the values of
of different 1-alkyl-3-methylimidazolium ([Cnmim]+, n is the number of C in the alkyl side chain – 2, 4, 6 or 8) TFSI− based ionic liquids (ILs) in γ-butyrolactone (GBL) are determined using the low concentration Chemical Model (lcCM). Based on the values of 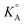 for ILs in GBL and earlier physicochemical systematic investigations in that solvent, the system with the lowest
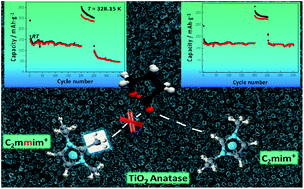
for ILs in GBL and earlier physicochemical systematic investigations in that solvent, the system with the lowest 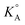 was selected for the preparation of the LiTFSI/C2mimTFSI/GBL electrolyte for testing TiO2 nanotube arrays as anode material for LIBs. In an attempt to realize LIBs with enhanced safety, we report herein a comparative study of the electrochemical properties of LiTFSI/C2mimTFSI/GBL and an electrolyte containing an IL without acidic C(2)H on the imidazolium cation, namely, LiTFSI/C2mmimTFSI/GBL. The presence of GBL can improve the reduction stability of imidazolium-based ILs and GBL in LiTFSI/IL/GBL-based electrolytes. It was shown that TiO2 nanotube structures display stable galvanostatic cycling in the LiTFSI/C2mimTFSI/GBL electrolyte after 350 full (dis-)charge cycles and after cell exposure to T = 328.15 K.
was selected for the preparation of the LiTFSI/C2mimTFSI/GBL electrolyte for testing TiO2 nanotube arrays as anode material for LIBs. In an attempt to realize LIBs with enhanced safety, we report herein a comparative study of the electrochemical properties of LiTFSI/C2mimTFSI/GBL and an electrolyte containing an IL without acidic C(2)H on the imidazolium cation, namely, LiTFSI/C2mmimTFSI/GBL. The presence of GBL can improve the reduction stability of imidazolium-based ILs and GBL in LiTFSI/IL/GBL-based electrolytes. It was shown that TiO2 nanotube structures display stable galvanostatic cycling in the LiTFSI/C2mimTFSI/GBL electrolyte after 350 full (dis-)charge cycles and after cell exposure to T = 328.15 K.


 Please wait while we load your content...
Please wait while we load your content...