Protonation of N2O and NO2 in a solid phase†
Abstract
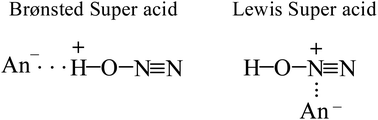
Adsorption of gaseous N2O on the acidic surface Brønsted centers of the strongest known solid acid, H(CHB11F11), results in formation of the N≡N–OH+ cation. Its positive charge is localized mainly to the H-atom, which is H-bonded to the CHB11F11− anion forming an asymmetric proton disolvate of the L1–H+⋯L2 type, where L1 = N2O and L2 = CHB11F11−. NO2 protonation under the same conditions leads to the formation of the highly reactive cation radical NO2H˙+, which reacts rapidly with an NO2 molecule according to the equation N2OH+ + NO2 → [N2O4H+] → N2OH+ + O2 resulting in the formation of two types of N2OH+ cations: (i) a typical Brønsted superacid, N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–OH+, with a strongly acidic OH group involved in a rather strong H-bond with the anion, and (ii) a typical strong Lewis acid, N
N–OH+, with a strongly acidic OH group involved in a rather strong H-bond with the anion, and (ii) a typical strong Lewis acid, N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N+–OH, with a positive charge localized to the central N atom and ionic interactions with the surrounding anions via the charged central N atom.
N+–OH, with a positive charge localized to the central N atom and ionic interactions with the surrounding anions via the charged central N atom.


 Please wait while we load your content...
Please wait while we load your content...