Defluorination and covalent grafting of fluorinated graphene with TEMPO in a radical mechanism†
Abstract
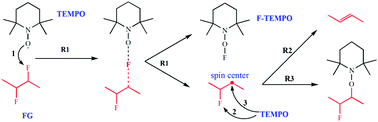
Fluorinated graphene (FG) can be regarded as the representative two-dimensional (2D) material to study the characteristics of “2D chemistry”, whereas its derivative reaction mechanism is still required to be revealed for the destination of deciduous fluorine atoms after defluorination of FG. Herein, we proposed a particular derivative reaction of FG by employing 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) as the attacking reagent, and the products were characterized via Electron Paramagnetic Resonance Spectroscopy (EPR), Mass Spectrometry (MS) and X-ray photoelectron spectroscopy (XPS). It was demonstrated that the defluorination caused by TEMPO occurred in a radical mechanism, thus leading to formations of new spin centers on graphene nanosheets as well as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. More importantly, the deciduous fluorine atoms after defluorination, which existed in TEMPO fluoride molecules, have been detected for the first time. Meanwhile, some TEMPO molecules were covalently grafted on the nanosheet, which resulted from the coupled reaction between TEMPO radical and the spin center on the FG nanosheet. These findings deepen the research of derivative reactions of FG, meanwhile providing a particular view to investigate the chemistry characteristics of 2D materials from a radical mechanism.
C bonds. More importantly, the deciduous fluorine atoms after defluorination, which existed in TEMPO fluoride molecules, have been detected for the first time. Meanwhile, some TEMPO molecules were covalently grafted on the nanosheet, which resulted from the coupled reaction between TEMPO radical and the spin center on the FG nanosheet. These findings deepen the research of derivative reactions of FG, meanwhile providing a particular view to investigate the chemistry characteristics of 2D materials from a radical mechanism.


 Please wait while we load your content...
Please wait while we load your content...