Highly-dispersed TiO2 nanoparticles with abundant active sites induced by surfactants as a prominent substrate for SERS: charge transfer contribution†
Abstract
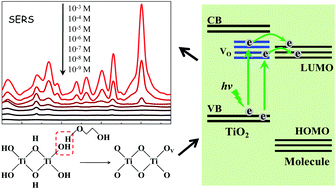
In this study, highly-dispersed TiO2 nanoparticles (NPs) with abundant active sites were synthesized by a simple sol-hydrothermal method with the assistance of surfactant polyethylene glycol (PEG) which served as an effective SERS-active substrate for the first time. The observed considerable SERS enhancement of 4-mercaptobenzoic acid (4-MBA) probe molecules on TiO2 NPs is attributed to the contribution of the charge transfer mechanism from the substrate to the probe molecule. It is suggested that PEG can act as a protective agent in the reductive calcination of the surfactant-coated nanocrystallite and consequently brings about abundant surface oxygen vacancies for TiO2 NPs, which provide more effective sites for the adsorption of probe molecules and promote the charge transfer effect and its consequent SERS enhancement. Moreover, the prepared TiO2 NPs can serve as an effective substrate for highly sensitive detection of p-aminobenzoic acid (PABA). The detection limit of PABA is as low as 1 × 10−8 M, which is the highest sensitivity among the reported determination methods. And, it proves that the prepared TiO2 substrate with abundant active sites is characterized by high stability, which offers a unique long service lifetime for SERS detection without losing its activity.


 Please wait while we load your content...
Please wait while we load your content...