Immobilization of alkynyl functionalized manganese phthalocyanine via click electrochemistry for electrocatalytic oxygen evolution reaction
Abstract
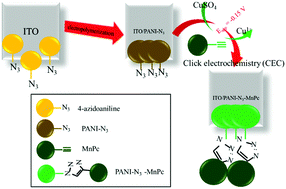
Peripherally and non-peripherally terminal alkynyl substituted manganese phthalocyanines (MnPc) were synthesized and characterized and then used as functional materials in modified electrodes. MnPcs were substituted with alkynyl groups, which are reactive moieties in click electrochemistry (CEC) reactions. Mn(III) cations were incorporated into the cavity of the Pc ring in order to increase the redox activity of the complexes. Electrochemical characterizations of the complexes were determined by voltammetric and in situ spectroelectrochemical measurements in order to determine their possible technological applications. MnPc complexes illustrated five redox couples and these redox couples were assigned as [Cl–MnIIIPc2−]/[Cl–MnIIPc2−]1−, [Cl–MnIIPc2−]1−/[Cl–MnIPc2−]2−, [Cl–MnIPc2−]2−/[ Cl–MnIPc3−]3−, and [Cl–MnIIIPc2−]/[Cl–MnIIIPc1−]1+ redox processes. The position of the substituents affected the mechanism of the redox reactions and influenced the tendency to react with the molecular oxygen. Moreover, changing the position of the substituents slightly influenced the peak potentials and reversibility of the redox processes. For the applications, modified electrodes (ITO/PANI-N3-MnPc and GCE/PANI-N3-MnPc) were constructed with CEC reaction between azido functionalized polyaniline (PANI-N3) and terminally alkynyl substituted MnPcs and these electrodes. Voltammetric characterizations of the modified electrodes illustrated suitable redox activity and conductivity for the practical applications. Finally, the GCE/PANI-N3-MnPc electrode was tested as a potential electrocatalyst for water splitting reaction. Although the GCE/PANI-N3-MnPc electrode did not catalyze the hydrogen evolution reaction (HER), it significantly catalyzed the oxygen evolution reaction (OER) in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...