A kinetic study of the CH2OO Criegee intermediate reaction with SO2, (H2O)2, CH2I2 and I atoms using OH laser induced fluorescence†
Abstract
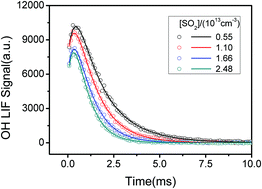
The OH laser induced fluorescence method was used to study the kinetics of CH2OO reacting with SO2, (H2O)2, CH2I2 and I atoms. Decay of CH2OO is not strictly first-order since its self-reaction is rapid. With this consideration, we derived the rate coefficient of CH2OO + SO2/(H2O)2/CH2I2/I taking into account the contribution of the CH2OO self-reaction. For the CH2OO + SO2 reaction, the rate coefficient is measured to be (3.88 ± 0.13) × 10−11 cm3 molecule−1 s−1 at 10 Torr, which agrees very well with a previously reported value obtained by directly monitoring CH2OO using the UV absorption method with the CH2OO self-reaction considered. We did not observe obvious evidence for SO2 catalysed CH2OO isomerization or the intersystem crossing effect in this reaction. CH2OO + (H2O)2 is supposed to account for the major sink of CH2OO in the atmosphere, but previous rate coefficient measurements were not in good agreement. We have revisited this reaction including the self-reaction of CH2OO and obtained the rate coefficient to be (7.53 ± 0.38) × 10−12 cm3 molecule−1 s−1 at 60 Torr and 300 K. The rate coefficients of CH2OO + CH2I2 and CH2OO + I were measured to be (5.2 ± 2.6) × 10−14 and (2.2 ± 1.1) × 10−12 cm3 molecule−1 s−1 respectively.


 Please wait while we load your content...
Please wait while we load your content...