Unveiling anomalous CO2-to-N2 selectivity of graphene oxide†
Abstract
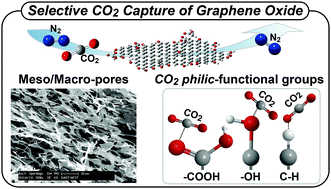
When dry sorbents are considered for CO2 capture, selective uptake of CO2 over other gases is highly desirable. However, most dry sorbents suffer from selectivity drop upon temperature increase. Here, we report that graphene oxide (GO) exhibits high CO2-to-N2 selectivity, and the selectivity rises anomalously with temperature increase; CO2-to-N2 selectivity that starts at 192 at 273 K increases to an extraordinarily high value of 607 at 323 K. These high values and unusual trends in selectivity are attributed to a combined effect of CO2-philicity from the functional groups of GO and its relatively large macropores that are efficient at releasing N2. In-depth analysis using FT-IR reveals CO2-philic electrostatic interactions where CO2 serves as an electron donor and acceptor simultaneously; while CO2 can bind with electron-rich oxygen-containing groups of GO, the oxygen of CO2 can also bind with hydrogen-containing groups at the edges of GO. The current study with GO highlights a design principle for highly selective CO2 capture represented by CO2-philic electrostatic sites coupled with relatively large pores for efficient N2 release.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...