Spin dependent interactions catalyse the oxygen electrochemistry
Abstract
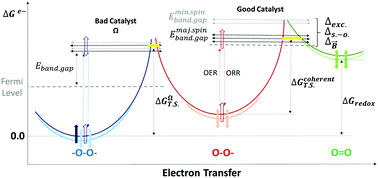
The technological interest of oxygen reduction and evolution reactions, ORR and OER, for the clean use and storage of energy has resulted in the discovery of multiple catalysts; and the physical and catalytic properties of the most active compositions are only comprehensible with the consideration of magnetic interactions. Spin dependent potentials via exchange interactions, spin–orbit coupling or through magneto-electric effects catalyse the oxygen electrochemistry. The best catalysts show metal sites with localized spins and electron delocalization; a correlation exists between the rate constant for charge transfer reactions and spin-dependent electron mobility. Since during the OER and ORR the number of unpaired electrons is not conserved, magnetic potentials in optimum catalysts act as selective gates to enhance the transport of local spin currents. Overall magnetic potentials can reduce the bonding properties of the, donor or acceptor, orbitals in the catalyst, and electrons more easily transfer over the conduction band. The influence of spin dependent forces is generally applicable to oxygen catalysis, and supplements the physical interactions relevant for inorganic or organic, electro or photo, artificial or natural processes.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...