Understanding the influence of low-frequency vibrations on the hydrogen bonds of acetic acid and acetamide dimers†
Abstract
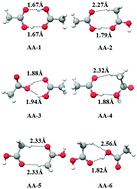
Low-frequency vibrations coupled to high-frequency modes are known to influence the hydrogen bond strengths in a weakly interacting dimer. In this context, various acetic acid and acetamide dimers were analyzed using Møller–Plesset second-order perturbation (MP2) and density functional theory (DFT)-based approaches with explicit anharmonicity corrections. The computed low-frequency fundamentals as well as the high-frequency modes, which were found to be related to hydrogen bonding (OH/NH stretching modes), were analyzed and their computed intensities were correlated with their hydrogen-bond strengths/binding energies. There are similarities in the nature of eight low-frequency fundamentals of these two dimers, and the in-plane bending and stretch-bend fundamentals of the different dimers of these two species (in this low-frequency region) have specific roles in their relative stability order. The computed linear correlations were further verified against the results from coupled cluster calculations including triple excitation (CCSD(T)), Gaussian-G4 (G4), Gaussian-G2-MP2 (G2MP2) and complete basis set (CBS-QB3) methods of high accuracy energy calculations. As a consequence of such linear correlations, an additive property of local fragment energies (responsible for hydrogen bonding) was found to be a valid approximation to predict the binding energies of such dimers and the idea was found to be extendable to the other homologues of these acids/amides.


 Please wait while we load your content...
Please wait while we load your content...