The catalytic effect of H2O on the hydrolysis of CO32− in hydrated clusters and its implication in the humidity driven CO2 air capture†
Abstract
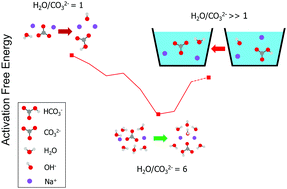
The hydration of ions in nanoscale hydrated clusters is ubiquitous and essential in many physical and chemical processes. Here we show that the hydrolysis reaction is strongly affected by relative humidity. The hydrolysis of CO32− with n = 1–8 water molecules is investigated using an ab initio method. For n = 1–5 water molecules, all the reactants follow a stepwise pathway to the transition state. For n = 6–8 water molecules, all the reactants undergo a direct proton transfer to the transition state with overall lower activation free energy. The activation free energy of the reaction is dramatically reduced from 10.4 to 2.4 kcal mol−1 as the number of water molecules increases from 1 to 6. Meanwhile, the degree of hydrolysis of CO32− is significantly increased compared to the bulk water solution scenario. Incomplete hydration shells facilitate the hydrolysis of CO32− with few water molecules to be not only thermodynamically favorable but also kinetically favorable. We showed that the chemical kinetics is not likely to constrain the speed of CO2 air capture driven by the humidity-swing. Instead, the pore-diffusion of ions is expected to be the time-limiting step in the humidity driven CO2 air capture. The effect of humidity on the speed of CO2 air capture was studied by conducting a CO2 absorption experiment using IER with a high ratio of CO32− to H2O molecules. Our result is able to provide valuable insights into designing efficient CO2 air-capture sorbents.


 Please wait while we load your content...
Please wait while we load your content...