External electric field control: driving the reactivity of metal-free azide–alkyne click reactions†
Abstract
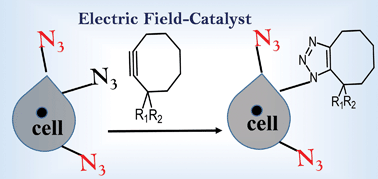
Recent reports have suggested that an external electric field (EEF) can assist and even control product selectivity. In this work, we have shown that the barrier for the Huisgen reaction between alkyl (aryl) azide and cyclooctyne(biflurocyclooctyne) is reduced by ∼3–4 kcal mol−1 when an oriented EEF is applied along the reaction axis. As a consequence of their inherently polar transition-states (TSs), a parallel orientation of the EEF results in enhancement of the charge transfer (CT) between the fragments and concomitant increase in the dipole moment along the reaction axes. This leads to an increase in the reaction rate for moderate EEFs in the range of 0.3–0.5 V Å−1. Since highly polar and directional environments are omnipresent in biological environments, metal-free click reactions can be further accelerated for non-invasive imaging of live-cells. Conceptually, electric field control appears to be a novel tool (catalyst) to drive, and possibly even tune, the reactivity of organic molecules.


 Please wait while we load your content...
Please wait while we load your content...