Increasing the lifetimes of charge separated states in porphyrin–fullerene polyads†
Abstract
Two linear polyads were designed using zinc(II)porphyrin, [ZnP], and N-methyl-2-phenyl-3,4-fullero-pyrrolidine (C60) where C60 is dangling either at the terminal position of [ZnP]–C6H4–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –C6H4–[ZnP]–C60 (1) or at the central position of [ZnP]–C6H4–
–C6H4–[ZnP]–C60 (1) or at the central position of [ZnP]–C6H4–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –C6H4–[ZnP(C60)]–C6H4–
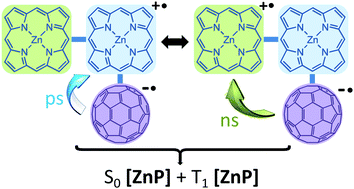
–C6H4–[ZnP(C60)]–C6H4–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –C6H4–[ZnP] (2) in order to test whether the fact of having one or two side electron donors influences the rate of electron transfer, ket. These polyads were studied using cyclic voltammograms, DFT computations, steady state and time-resolved fluorescence spectroscopy, and femtosecond transient absorption spectroscopy (fs-TAS). Photo-induced electron transfer confirmed by the detection of the charge separated state [ZnP˙+]/C60˙− from fs-TAS occurs with rates (ket) of 3–4 × 1010 s−1 whereas the charge recombinations (CRs) are found to produce the [ZnP] ground state via two pathways (central [ZnP˙+]/C60˙− (ps) and terminal central [ZnP˙+]/C60˙− (ns) producing [1ZnP] (ground state) and [3ZnP*]). The formation of the T1 species is more predominant for 2.
–C6H4–[ZnP] (2) in order to test whether the fact of having one or two side electron donors influences the rate of electron transfer, ket. These polyads were studied using cyclic voltammograms, DFT computations, steady state and time-resolved fluorescence spectroscopy, and femtosecond transient absorption spectroscopy (fs-TAS). Photo-induced electron transfer confirmed by the detection of the charge separated state [ZnP˙+]/C60˙− from fs-TAS occurs with rates (ket) of 3–4 × 1010 s−1 whereas the charge recombinations (CRs) are found to produce the [ZnP] ground state via two pathways (central [ZnP˙+]/C60˙− (ps) and terminal central [ZnP˙+]/C60˙− (ns) producing [1ZnP] (ground state) and [3ZnP*]). The formation of the T1 species is more predominant for 2.


 Please wait while we load your content...
Please wait while we load your content...