Structure and stability of small lithium-chloride LinClm(0,1+) (n ≥ m, n = 1–6, m = 1–3) clusters†
Abstract
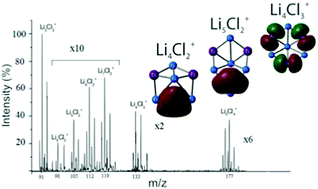
In the present study, we report the results of a detailed theoretical investigation along with the experimental observations of chlorine-doped small lithium clusters. The cluster ions of the type LinClm+ (n ≥ m, n = 1–6, m = 1–3) were obtained by the evaporation of LiCl from a Knudsen cell as a chemical reactor in the temperature range between 1800 and 2700 K. Heterogeneous clusters with more than one Cl atom are produced and detected for the first time, and the experimental conditions for formation and stability are examined. The structural characteristics and stabilities of neutral and positively charged LinClm species are analyzed by using quantum chemistry methods. Doping lithium clusters with chlorine increases their stability, although there is a typical closed-shell–open-shell alternation in stability. Calculated dissociation energies are the best indicator of cluster stability of experimentally detected clusters. Heterogeneous lithium-chloride clusters can be viewed as species consisting of m negative Cl− ions and a positively charged Lin(1+,2+) “cage”; upon ionization, an electron departs from the lithium cage. An important reason for the higher stability of closed-shell clusters is the delocalization of electrons over the lithium cage, which is more energetically favored than localization of electrons between two lithium atoms. According to their ionization energies, the titled clusters can be classified as “superalkalis”.


 Please wait while we load your content...
Please wait while we load your content...