Abstract
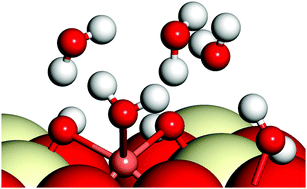
We report a detailed density functional theory (DFT) study in conjunction with extended X-ray absorption fine structure (EXAFS) experiments on the geometrical and local electronic properties of Cu adatoms and Cu(II) ions in presence of water molecules and of CuO nanoclusters on the CeO2(110) surface. Our study of (CuO)n(=1,2&4) clusters on CeO2(110) shows that based on the Cu–O environment, the geometrical properties of these clusters may vary and their presence may lead to relatively high localization of charge on the exposed surfaces. We find that in the presence of an optimum concentration of water molecules, Cu has a square pyramidal geometry, which agrees well with our experimental findings; we also find that Cu(II) facilitates water adsorption on the CeO2(110) surface. We further show that a critical concentration of water molecules is required for the hydrolysis of water on Cu(OH)2/CeO2(110) and on pristine CeO2(110) surfaces.


 Please wait while we load your content...
Please wait while we load your content...