The length but not the sequence of peptide linker modules exerts the primary influence on the conformations of protein domains in cellulosome multi-enzyme complexes
Abstract
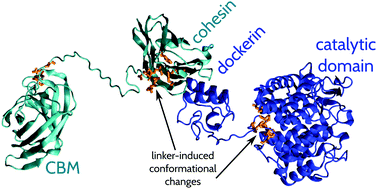
Cellulosomes are large multi-protein catalysts produced by various anaerobic microorganisms to efficiently degrade plant cell-wall polysaccharides down into simple sugars. X-ray and physicochemical structural characterisations show that cellulosomes are composed of numerous protein domains that are connected by unstructured polypeptide segments, yet the properties and possible roles of these ‘linker’ peptides are largely unknown. We have performed coarse-grained and all-atom molecular dynamics computer simulations of a number of cellulosomal linkers of different lengths and compositions. Our data demonstrates that the effective stiffness of the linker peptides, as quantified by the equilibrium fluctuations in the end-to-end distances, depends primarily on the length of the linker and less so on the specific amino acid sequence. The presence of excluded volume – provided by the domains that are connected – dampens the motion of the linker residues and reduces the effective stiffness of the linkers. Simultaneously, the presence of the linkers alters the conformations of the protein domains that are connected. We demonstrate that short, stiff linkers induce significant rearrangements in the folded domains of the mini-cellulosome composed of endoglucanase Cel8A in complex with scaffoldin ScafT (Cel8A-ScafT) of Clostridium thermocellum as well as in a two-cohesin system derived from the scaffoldin ScaB of Acetivibrio cellulolyticus. We give experimentally testable predictions on structural changes in protein domains that depend on the length of linkers.


 Please wait while we load your content...
Please wait while we load your content...