Less stable tautomers form stronger hydrogen bonds: the case of water complexes†
Abstract
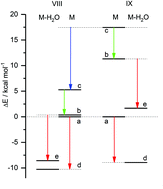
Hydrogen bonding in cyclic complexes of water with tautomeric pairs of molecules M0 and M1 is calculated to be stronger by more than 25% for the less stable tautomer M1 in all cases where the energy gap between the two tautomers is large (ΔE(M0 − M1) > 10 kcal mol−1). This is accompanied by a large red-shift (>200 cm−1) of the N–H/O–H stretch frequency in the complexes involving M1. Large barriers for double proton transfer in both directions should permit an experimental verification. Exceptions to this rule were found in heterocycles with an N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O fragment incorporated into a conjugated cycle resulting in two nearly degenerate tautomers – keto and enol forms. The wavefunction of the keto form has a large contribution from a zwitterionic VB structure which is also aromatic. This increases the polarity of the keto group, making the oxygen atom a strong H-bond acceptor. It can also stabilize the keto form below the aromatic enol form. In this case the extra-HB stabilization is observed for the most stable tautomer (i.e. for the keto form). H-bonding enhances the aromatic character of less aromatic molecules, but the more aromatic tautomers partially loose aromaticity.
O fragment incorporated into a conjugated cycle resulting in two nearly degenerate tautomers – keto and enol forms. The wavefunction of the keto form has a large contribution from a zwitterionic VB structure which is also aromatic. This increases the polarity of the keto group, making the oxygen atom a strong H-bond acceptor. It can also stabilize the keto form below the aromatic enol form. In this case the extra-HB stabilization is observed for the most stable tautomer (i.e. for the keto form). H-bonding enhances the aromatic character of less aromatic molecules, but the more aromatic tautomers partially loose aromaticity.


 Please wait while we load your content...
Please wait while we load your content...