On the bulk transport process and its impact on the electrode behavior of mixed conducting electrodes for SOFCs
Abstract
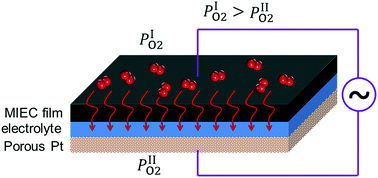
The surface reaction and bulk transport processes represent two pillars in the kinetics studies of SOFC electrodes. The driving force of the ion transport process in the mixed ion-electron conductor (MIEC) electrodes is recapitulated. Qualitative and quantitative analyses are conducted on the so-called “bulk diffusion” process, along with experimental verification through the (LaSr)(CoFe)O3−δ cathode. It is proven that an electrical field will be present inside the MIEC electrode, which drives the motion of ions along with the concentration gradient. In the steady state, the distributions of ce, cv, ϕ, etc. are obtained. The impact of this electrical field on the electrode kinetics and stability is discussed. It is revealed that: (a) the equivalent PO2 at the interface calculated according to the overpotential and the Nernst equation could overestimate the stability concern. (b) From the viewpoint of the bulk pathway, polarization is not necessarily a beneficial factor, which could open up, or shut down the bulk pathway depending on the ionic defect type. (c) The electrical field can also drive the motion of cations, leading to composition evolution near the interface in materials with relatively rich cation vacancies.


 Please wait while we load your content...
Please wait while we load your content...