Rendering cross-conjugated azophenine derivatives emissive to probe the silent photophysical properties of emeraldine†
Abstract
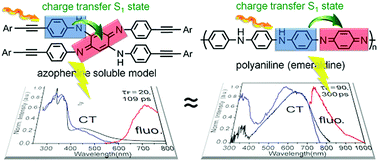
An azophenine derivative was synthesized by coupling truxene and azophenine via the copper-free Sonagashira reaction using Pd2(dba)3 and As(PPh)3 as catalysts. The crystal structure of this heavy azophenine model (∼4000) was made and the identity of the structure was confirmed. By introducing truxene groups into this cross-conjugated structure, the deactivating rotations around the NH–C6H4 bonds were slowed down, which rendered this derivative near-IR emissive at 298 K. This species provided then the appropriate spectral and kinetic signatures for knowing where and what to look for in emeraldine, which was called non-emissive. Besides, two other compounds were also synthesized as models for this azophenine derivative for comparison and interpretation purposes.


 Please wait while we load your content...
Please wait while we load your content...