A new insight into the thermodynamical criterion for the preparation of semiconductor and metal nanocrystals using a polymerized complexing method†
Abstract
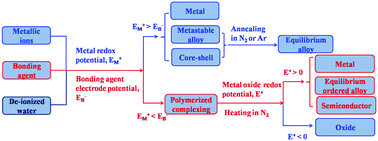
This work reports the intrinsic thermodynamical criterion for the preparation of metal and semiconductor nanocrystals using a polymerized complexing method. The basic principle of this method is the formation of a polymerized complexing structure between mono-, binary-, or ternary-metallic ions and bonding agents in aqueous or ethylene glycol solutions by evaporation of the solvents. Heat treatment of the complexing structure under N2 atmosphere produces H2 and CH4 gases, which can reduce the oxide crystalline nuclei to semiconductor and metallic nanocrystals. Experimental results show that Te, CdTe, Ag2Te, CuTe, NiTe1.5, CoTe1.5, Bi2Te3, Sb2Te3, Bi–Sb, In2Te3, Ni2.9SnTe2, CuGaTe2, and CuInS2 semiconductors and Bi, Sb, Ag, Cu, and Ni metallic nanocrystals can be prepared by this method. Transmission electron microscopy observations show that the obtained Bi, Ag2Te, Bi2Te3, Sb2Te3, CdTe, and NiTe1.5 nanocrystals have grain sizes in the nanometer range. The types of metallic and semiconductor phase that can be obtained by this method are explained by the thermodynamical criterion based on calculations of the Gibbs free energy and electrode potential. It is proposed that the crystalline phase of the final product is controlled by the change of the Gibbs free energies of the reactions of the metal oxide with reducing gases and the metal oxide redox electrode potentials, not the metal redox standard electrode potentials and electronegativities of the elements. Furthermore, a prediction is presented for the preparation of other kinds of binary and ternary compound based on the thermodynamical criterion. Our results provide new insight into facile and green preparation of semiconductor and metal nanocrystals.


 Please wait while we load your content...
Please wait while we load your content...