Does increasing pressure always accelerate the condensed material decay initiated through bimolecular reactions? A case of the thermal decomposition of TKX-50 at high pressures
Abstract
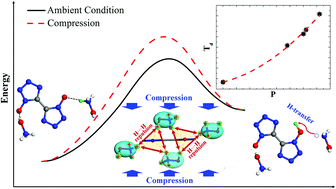
Performances and behaviors under high temperature–high pressure conditions are fundamentals for many materials. We study in the present work the pressure effect on the thermal decomposition of a new energetic ionic salt (EIS), TKX-50, by confining samples in a diamond anvil cell, using Raman spectroscopy measurements and ab initio simulations. As a result, we find a quadratic increase in decomposition temperature (Td) of TKX-50 with increasing pressure (P) (Td = 6.28P2 + 12.94P + 493.33, Td and P in K and GPa, respectively, and R2 = 0.995) and the decomposition under various pressures initiated by an intermolecular H-transfer reaction (a bimolecular reaction). Surprisingly, this finding is contrary to a general observation about the pressure effect on the decomposition of common energetic materials (EMs) composed of neutral molecules: increasing pressure will impede the decomposition if it starts from a bimolecular reaction. Our results also demonstrate that increasing pressure impedes the H-transfer via the enhanced long-range electrostatic repulsion of H+δ⋯H+δ of neighboring NH3OH+, with blue shifts of the intermolecular H-bonds. And the subsequent decomposition of the H-transferred intermediates is also suppressed, because the decomposition proceeds from a bimolecular reaction to a unimolecular one, which is generally prevented by compression. These two factors are the basic root for which the decomposition retarded with increasing pressure of TKX-50. Therefore, our finding breaks through the previously proposed concept that, for the condensed materials, increasing pressure will accelerate the thermal decomposition initiated by bimolecular reactions, and reveals a distinct mechanism of the pressure effect on thermal decomposition. That is to say, increasing pressure does not always promote the condensed material decay initiated through bimolecular reactions. Moreover, such a mechanism may be feasible to other EISs due to the similar intermolecular interactions.


 Please wait while we load your content...
Please wait while we load your content...