On kinetic modelling for solar redox thermochemical H2O and CO2 splitting over NiFe2O4 for H2, CO and syngas production
Abstract
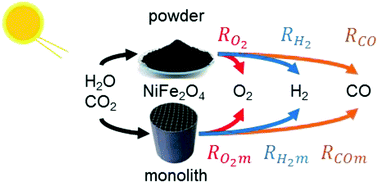
This study aims at developing a kinetic model that can adequately describe solar thermochemical water and carbon dioxide splitting with nickel ferrite powder as the active redox material. The kinetic parameters of water splitting of a previous study are revised to include transition times and new kinetic parameters for carbon dioxide splitting are developed. The computational results show a satisfactory agreement with experimental data and continuous multicycle operation under varying operating conditions is simulated. Different test cases are explored in order to improve the product yield. At first a parametric analysis is conducted, investigating the appropriate duration of the oxidation and the thermal reduction step that maximizes the hydrogen yield. Subsequently, a non-isothermal oxidation step is simulated and proven as an interesting option for increasing the hydrogen production. The kinetic model is adapted to simulate the production yields in structured solar reactor components, i.e. extruded monolithic structures, as well.


 Please wait while we load your content...
Please wait while we load your content...