Infrared study on the thermal evolution of solid state formamide
Abstract
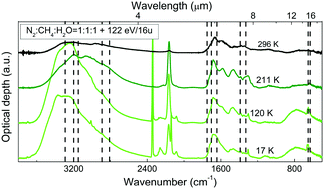
Laboratory experiments have shown that the energetic processing, i.e. ion bombardment and UV photolysis, of interstellar grain mantles and cometary surfaces is efficient in the production of formamide. To explain its presence in the gas-phase in these astrophysical environments, a desorption mechanism has to be taken into account. In this work we show experimental results on the thermal evolution of formamide when deposited at 17 K as pure and in mixture with water or carbon monoxide. In these samples, we observed formamide desorption at 220 K. Moreover, we discuss its synthesis in a mixture containing molecular nitrogen, methane and water (N2:CH4:H2O) deposited at 17 K and bombarded with 200 keV H+. Heating the sample, we observed that the newly formed formamide remains trapped in the refractory residue produced after the ion bombardment up to 296 K. To analyse the samples we used Fourier transform-infrared spectroscopy (FT-IR) that allowed us to study the infrared spectra between the deposition and the complete desorption of formamide. Here we discuss the experimental results in view of their astrophysical relevance.


 Please wait while we load your content...
Please wait while we load your content...