New insights into water photooxidation on reductively pretreated hematite photoanodes†
Abstract
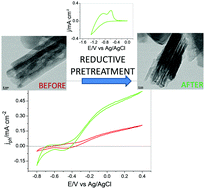
It has been recently demonstrated that the photoactivity toward oxygen evolution of a number of n-type metal oxides can be substantially improved by a reductive electrochemical pretreatment. Such an enhancement has been primarily linked to the formation of low valent metal species that increase electrode conductivity. In this work, we report new insights into the electrochemical doping using highly ordered (110)-oriented hematite nanorods directly grown on FTO. The reductive pretreatment consists in applying negative potentials for a controlled period of time. Such a pretreatment was optimized in both potentiostatic and potentiodynamic regimes. We show that the optimized pretreatment enhances electrode conductivity due to an increase in charge carrier density. However, it additionally triggers changes in the morphologic, catalytic and electronic properties that facilitate the separation and collection of the photogenerated charge carriers causing an up to 8-fold enhancement in the photocurrent for water oxidation. The reductive pretreatment can be considered as a highly controllable electrochemical n-type doping with the amount of generated Fe2+/polaron species and the change in film morphology as the main factors determining the final efficiency for water photooxidation of the resulting electrodes.


 Please wait while we load your content...
Please wait while we load your content...