The VN3H defect in diamond: a quantum-mechanical characterization†
Abstract
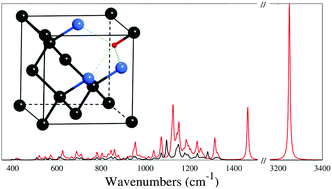
The VN3H defect in diamond (a vacancy surrounded by three nitrogen and one carbon atoms, the latter being saturated by a hydrogen atom) is investigated quantum-mechanically by use of a periodic supercell approach, an all-electron Gaussian-type basis set, “hybrid” functionals of density functional theory, and the Crystal program. Three fully optimized structural models (supercells containing 32, 64, and 128 atoms) are considered to investigate the effect of defect concentration. The electronic configuration of the defect is reported along with a description of its structural features. In particular, the influence of the lone-pair electrons of the three nitrogen atoms on the C–H bond is discussed. A thorough characterization of the vibrational spectroscopic features of the VN3H defect is also presented, where the anharmonicity of the most relevant normal modes is discussed. The infrared and Raman spectra show specific peaks, which allow for the identification of this particular defect among the many defects that are commonly present in both natural and irradiation-damaged diamonds. In particular, the main feature of the spectral fingerprint of the defect (i.e. the C–H stretching mode), experimentally observed at 3107 cm−1, is here computed at 3094 cm−1 with the B3LYP “hybrid” functional (with an anharmonic redshift of 157 cm−1 with respect to its harmonic value). The role played by the three nitrogen atoms on the spectral features of the defect is clearly identified through the redshift due to the 14N → 15N isotopic substitution.


 Please wait while we load your content...
Please wait while we load your content...