Analysing the microenvironment of 2-p-toluidinylnaphthalene-6-sulfonate (TNS) in solvents and in different conformational states of proteins in relation to its fluorescence properties: a computational study†
Abstract
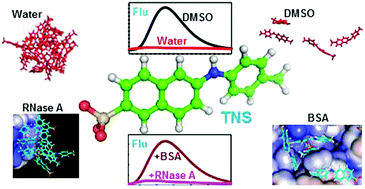
Characterization of different conformational states of proteins is essential to understand their stability and activity. Biophysical techniques aid in analysing these conformational states and molecular fluorescence is one of the most reliable and quickly accessible methods. Apart from the intrinsic fluorescence of proteins, external fluorescence dyes such as TNS, ANS, nile red and thioflavin are also used to characterize partially unfolded, aggregated and fibrillar states of proteins, though their exact molecular-level interactions with proteins are yet to be completely unravelled. The present study attempts to investigate the binding of TNS molecules on different conformational states of proteins. Unconstrained molecular dynamics simulation of 50 molecules of TNS with the native state of BSA, native and two partially unfolded states of RNase A and α-lactalbumin in water was carried out. Dynamics simulation of TNS alone in different solvents such as water, ethanol, DMF and DMSO was also performed. Binding environments in all the proteins and the solvents were analysed in terms of H-bonding interactions, order of contacts, amino acid specificity and conformational changes of TNS, and correlated with experimentally observed fluorescence changes of the dye. The results suggest that TNS forms aggregates in water whereas in non-aqueous solvents the order of aggregates is lower which might result in an enhancement of its fluorescence intensity. Further, TNS preferably interacts with basic and aromatic amino acid residues of the proteins. In RNase A and α-lactalbumin, most of the TNS molecules tend to form aggregates even with the unfolded conformations of the proteins. However in BSA, the number of aggregated TNS molecules is less and TNS molecules in monomeric form are found in the hydrophobic crevices of the protein. This might result in an enhancement of the fluorescence in BSA compared to the other proteins. The distributions of angles and dihedrals of TNS in different environments suggest that the bending movement between the naphthyl and tolyl rings is constrained whereas significant planar rotations could be observed both in solvents and in protein-bound states.


 Please wait while we load your content...
Please wait while we load your content...