Helium-3 gas self-diffusion in a nematically ordered aerogel at low temperatures: enhanced role of adsorption
Abstract
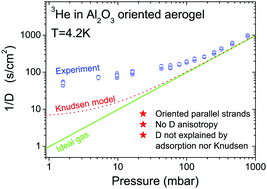
We performed 3He gas diffusion measurements for the first time in a highly porous ordered Al2O3 aerogel sample at a temperature of 4.2 K using a nuclear magnetic resonance field gradient technique. A strong influence of 3He adsorption in the aerogel on self-diffusion is observed. The classical consideration of adsorptive gas diffusion in mesopores leads to anomalously high tortuosity factors. The application of a more sophisticated model than the simple combination of empirical two-phase diffusion and the Knudsen gas diffusion models is required to explain our results. Anisotropic properties of the aerogel are not reflected in the observed gas diffusion even at low gas densities where the anisotropic Knudsen regime of diffusion is expected. The observed gas densification indicates the influence of the aerogel attractive potential on the molecular dynamics, which probably explains the reduced diffusion process. Perhaps this behavior is common for any adsorptive gases in nanopores.


 Please wait while we load your content...
Please wait while we load your content...